Microporous poly(D,L-lactide) acid-carbon nanodots nanocomposite scaffolds with multicolor emission for image-guided bone regeneration
Nicolò Mauro, Giovanna Calabrese, Alice Sciortino, Fabrizio Messina, Gaetano Giammona, Gennara Cavallaro

Significant bone defects arising from trauma, congenital anomalies, and diseases like cancer present formidable challenges for surgeons. In answer to this demand, bone tissue engineering has emerged as a promising approach in recent years, entailing the strategic integration of osteogenic progenitor cells, growth factors, and porous biocompatible scaffolds. An optimal scaffold should possess key properties, such as biocompatibility and porosity to sustain cell proliferation and differentiation, together with imaging contrast (e.g. fluorescence) which allows monitoring in vivo [1]. Additionally, it should exhibit osteoconductivity and osteoinductivity to facilitate the bone healing process. Moreover, biodegradability is crucial to enable gradual restoration by the host tissue. Poly(D,L-lactide) acid (PLA) has gained attention for its good mechanical properties, degradation rate, and ease of processing. Besides, carbon dots (CDs), a class of 0- D carbon allotrope, have also emerged as intriguing, finding applications in various biological contexts due to their high specific surface area, chemical stability, biocompatibility, low toxicity, antimicrobial properties, and biodegradability. Additionally, CDs exhibit versatile features such as stable fluorescence. These unique attributes position CDs as ideal materials for engineering fluorescent scaffolds, trackable by in vivo imaging, demonstrating excellent outcomes in wound healing and bone regeneration. In this study, we used heterophase melt-extrusion transesterification (HMET) to obtain a PLA-CD nanocomposite containing 1% multicolor CDs, and we employed this nanocomposite as a starting material for the production of microporous PLA-CD scaffolds with tailor-made properties by using the temperature-induced phase separation (TIPS) technique [2]. In a nutshell, CDs of 5.3 ± 0.4 nm in diameter exhibiting tunable multicolor emission (ranging from blue-QY 12% to NIR-QY 1%) and distinct surface polar groups exploitable for subsequent surface functionalization, including hydroxyl, were used to obtain a PLA nanocomposite by one-pot HMET with PLA chains providing exceptional thermal stability. The resultant PLA-CDs nanocomposite was used to produce microporous scaffolds with bright fluorescence in the solid state, interconnected pores, and robust structure by a rapid cooling of a dispersion of PLA-CDs in dioxane/water (TIPS) (Fig. 1).
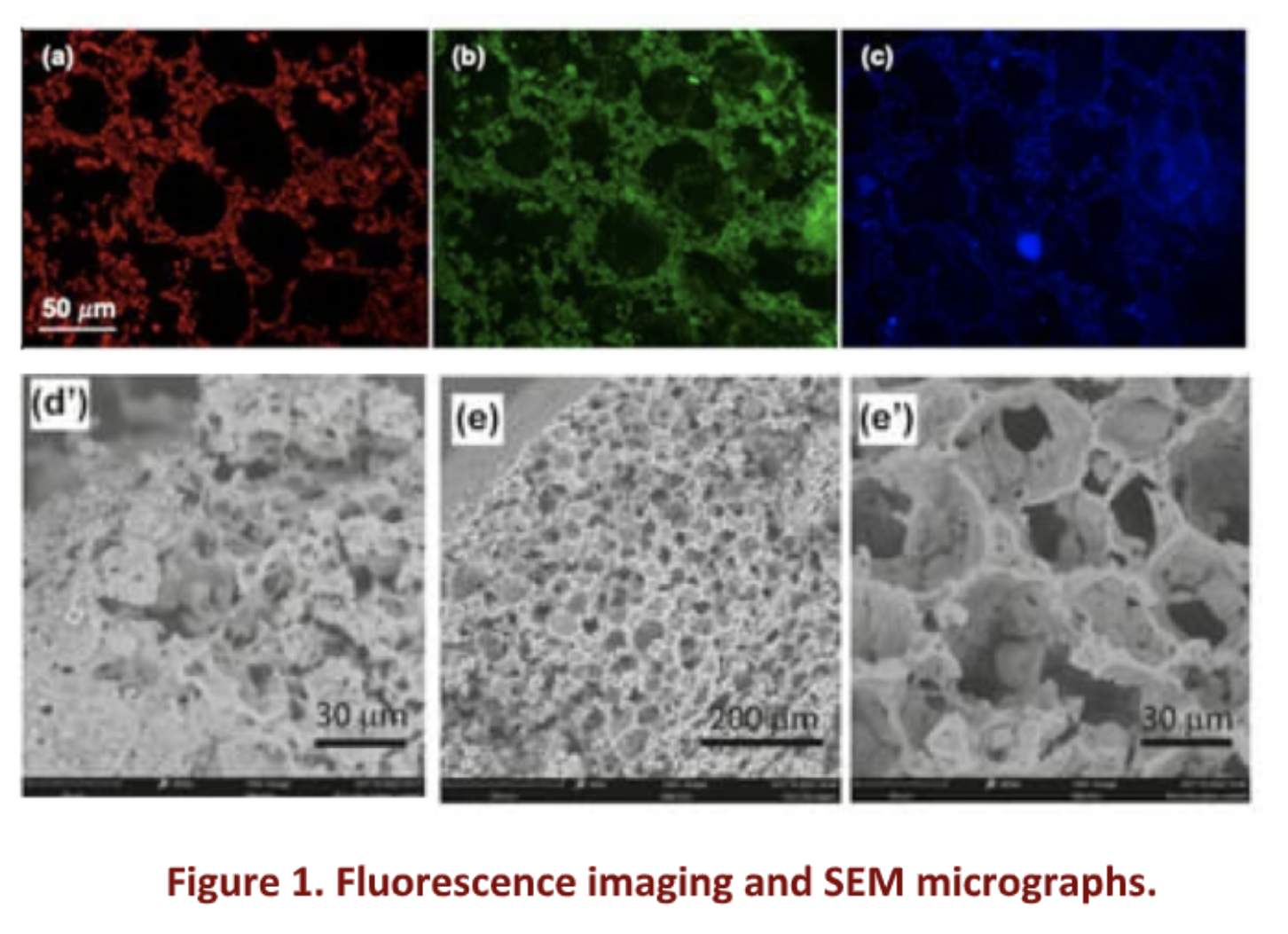
The presence of CDs homogeneously dispersed in the PLA matrix also accelerates PLA degradation as a function of the amount of CDs employed. Moreover, the storage modulus (G’) of the PLA-CDs was about two orders of magnitude higher (810 7 vs 510 5 Pa) compared to the bare PLA, implying strong interactions between the PLA chains on the CDs surface and the matrix on the whole. Biocompatibility assessments involved studying human adipose-derived stem cell (hADSC) growth on PLA-CDs scaffolds, validated through analyses of cell viability and morphology. H&E staining was conducted to assess hADSC penetration and propagation throughout the entire PLA-CD 5% scaffolds. Results after 21 days of culture showed cells present in all analyzed sections, indicating strong infiltration and colonization within the scaffold structure, and positioning it as a compelling choice for the advanced production of biomedical devices.
References
- D.W. Hutmacher, M. Sittinger, M.V. Risbund Trends Biotechnol. 2004, 22, 354.
- N. Mauro, G. Calabrese, A. Sciortino, M.G. Rizzo, F. Messina, G. Giammona, G. Cavallaro Materials 2024, 17, 449.