Thermal and mechanical properties of furan based thermoplastics and biobased epoxy thermosets
University Côte d’Azur, Institute of Chemistry of Nice, UMR CNRS 7272, 06100 Nice, France

Biobased polymers constitute a promising alternative to replace their petrosourced counterparts. However, they sometimes exhibit more complex behaviors as well as lower thermal and mechanical properties. The crystallization rate of semi-crystalline polymers as well as the polymerization kinetics of thermosetting resins have a major impact on the final properties of the material. Understanding these phenomena is therefore a crucial element for obtaining optimal properties.
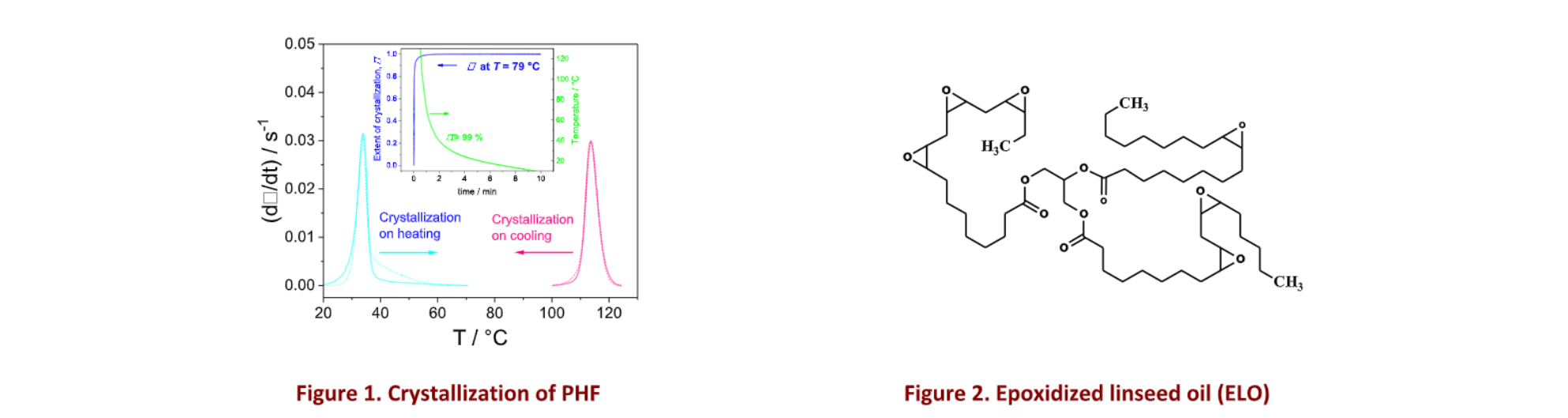
The presentation will start with the study of the crystallization of a promising flexible biobased semi-crystalline thermoplastic polyester, the poly(hexamethylene 2,5-furan dicarboxylate) (PHF) [ 1 ]. The nonisothermal crystallization from the melt and from the glass was studied by means of DSC, Fast Scanning Calorimetry and advanced kinetic analysis. It is shown that changes in the crystallization regime occurs at certain temperatures (i. e. 127.5, 121, 110 and 105 °C for crystallization from the melt, and 30 and 39 °C for crystallization from the glass) and that the end of the crystallization presents some deviation from what is predicted by the Hoffman-Lauritzen’s theory of growth rate. A method is presented that allows the determination of the equilibrium melting temperature, the infinite glass transition temperature, the temperature at maximum of the growth rate, and accurate simulation of crystallization rate curves [ 2 , 3 , 4 ] (Fig.1).
The second example will concern the use of low viscosity eutectic mixtures to use carboxilic acids as hardeners for biobased thermosets [ 5 , 6 ]. Natural carboxylic acids are a potential alternative as they are derived from plants and their multifunctionality provide the possibility for crosslinking with biobased epoxy resins, but their use as crosslinkers is limited by their high melting temperature. Diethyl malonate (DEM), diethyl succinate (DES), diethyl glutarate (DEG), diethyl oxalate (DEO) and triethyl citrate (TEC), were mixed with carboxilic acids to make efficient eutectic hardeners (EH) by decreasing the melting temperature and viscosity. Then, the EH were used to cure epoxidized linseed oil (ELO) (Fig.2). Polymerization mechanism was studied using advanced kinetic analysis and the cured materials were characterized by DMTA and mechanical tests. A new method based on a two-step model for crosslinking polymerization is presented [ 7 ]. The model appears to fit all data very well and yield physically meaningful parameters. The model is applicable to any processes that involve the interplay between the reaction and diffusion kinetics.
References
- G. Z. Papageorgiou, V. Tsanaktsis, D. G. Papageorgiou, K. Chrissafis, S. Exarhopoulos, D. N. Bikiaris Eur. Polym. J. 2015, 67, 383. 2 . A. Codou, N. Guigo, J. van Berkel, E. de Jong, N. Sbirrazzuoli Macromol. Chem. Phys. 2014, 215, 2065.
- N. Guigo, J. van Berkel, E. de Jong, N. Sbirrazzuoli Thermochim. Acta 2017, 650, 66.
- N. Guigo, G. Z. Papageorgiou, N. Poulopoulou, D. N. Bikiaris, N. Sbirrazzuoli Polymer 2023, 285, 126366.
- J. Tellers, P. Willems, B. Tjeerdsma, N. Guigo, N. Sbirrazzuoli Green Chem. 2020, 22, 3104.
- J. Tellers, M. Jamali, P. Willems, B. Tjeerdsma, N. Sbirrazzuoli, N. Guigo Green Chem. 2021, 23, 536.
- N. Sbirrazzuoli, S. Vyazovkin Chem. Eng. J. 2024, 481, 148414.