What are hydrogels?
Hydrogels are 3-dimensional networks of hydrophilic polymers, either natural or synthetic.

Networks can be formed by chemical or physical cross-linking of individual macromolecules. The main feature of hydrogels is that they are able to absorb large amounts of water without dissolving.
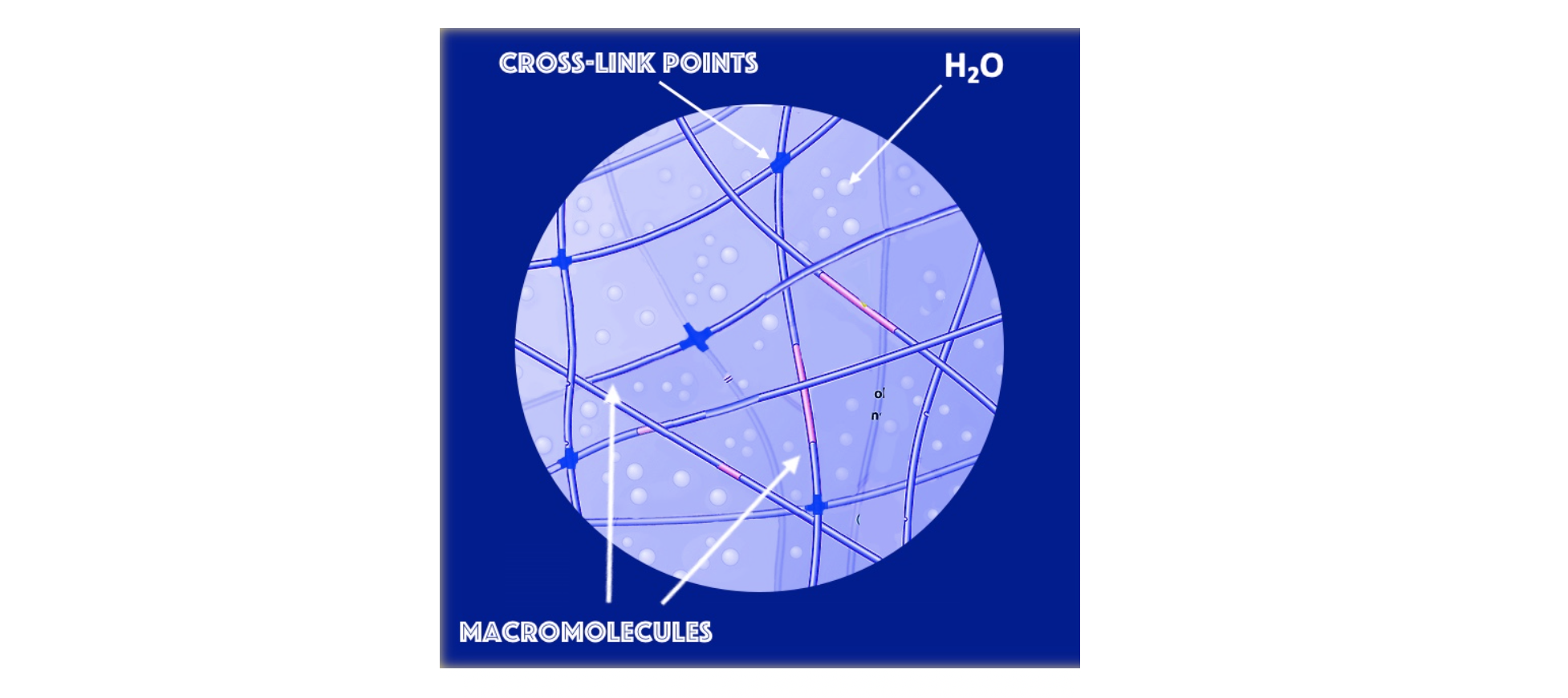
Figure 1: 3-dimensional hydrogel network.
What are the main properties of hydrogels?
Hydrogels are soft and elastic materials that can be easily processed into different shapes.

Figure 2: Hydrogel samples of different shape.
Hydrogels can normally undergo significant volume changes or gel-sol phase transitions in response to specific physical or chemical stimuli. The physical stimuli include temperature, electric and magnetic fields, solvent composition, light intensity and pressure, whereas the chemical stimuli include pH, ionic strength and specific chemical compositions. The response of hydrogels to external stimuli is normally reversible and they are capable of returning to their initial state after a reaction as soon as the trigger is removed.
What are the main applications of hydrogels?
The first relevant application of hydrogels was the production of contact lenses. Many other important applications have followed, including as components of drug delivery systems, dyes and heavy metal ions removal, scaffolds for tissue engineering, pH sensors.