What does young carbon mean?
Young carbon is a term used in contrast to fossil carbon, present in fossil fuels, which is millions of years old. Young carbon is indeed produced by plants that grew relatively recently comparing to fossil fuels.
How can young carbon and fossil carbon be distinguished?
Young carbon and fossil carbons can only be distinguished by means of carbon-14 dating. Carbon-14, or 14C, is a radiocarbon, as it is the radioactive carbon isotope. It is well-known for its use in radiocarbon dating of fossils and of archeological artifacts, including mummies and cave drawings. The radioactive decay of 14C is called beta decay and in it one of the neutrons of carbon is converted into a proton. Hence carbon is converted into the non-radioactive nitrogen-14. The half-life of 14C, that is, the time necessary to halve its concentration, is 5.700 years.
When can we use 14C as a tracer?
14C is very useful for monitoring the emission of carbon dioxide produced by the combustion of fossil fuels.
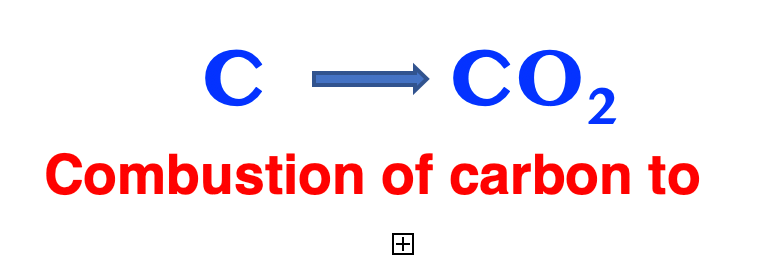
Carbon atoms present in fossil fuels are millions of years old and no longer contain the 14C isotope initially present, as it has decayed to non-detectable levels. Therefore, carbon dioxide, CO2, derived from the combustion of fossil fuels is 14C-free. Conversely, carbon dioxide derived from the combustion of young sources, namely from burning of plants, still contains the 14C radioisotope. This makes 14C an ideal tracer to understand whether carbon dioxide in the atmosphere comes from the combustion of fossil fuels or of young carbon.