Synergism between α-amino acid-derived polyamidoamines and sodium montmorillonite for enhancing the flame retardancy of cotton fabrics
Polyamidoamine (PAA)/sodium montmorillonite (MMT) nanocomposite coatings were investigated as flame retardants for cotton to ascertain whether the addition of clay improved the efficacy of PAAs thanks to its ability to act as insulating shield during combustion.
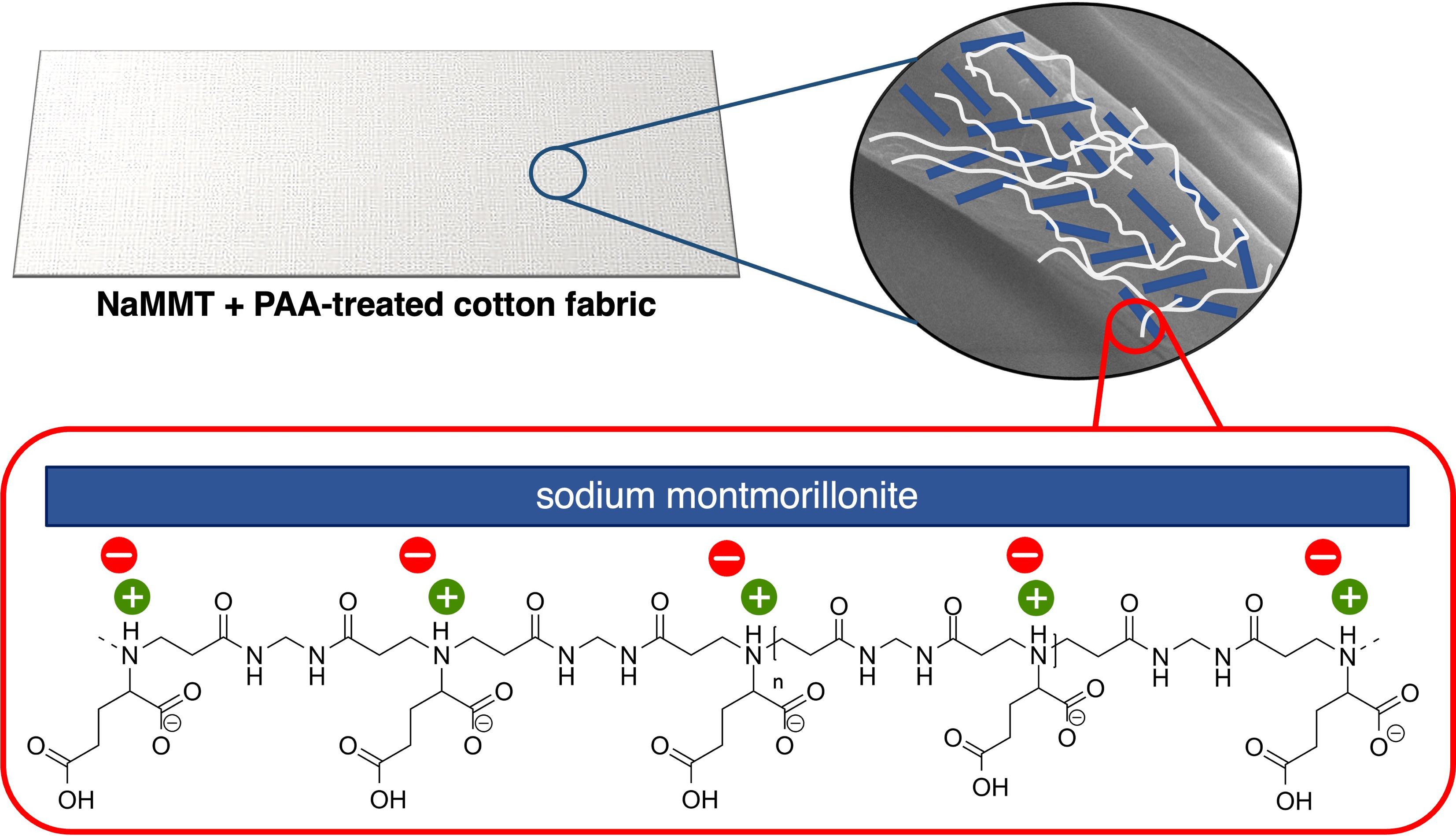
Three amphoteric PAAs were obtained by reacting N,N’-methyl- enebisacrylamide with natural α-amino acids, namely glycine (M-GLY), arginine (M-ARG) and glutamic acid (M- GLU). These PAAs act as intumescent flame retardants for cotton performing well in horizontal flame spread tests (HFSTs) but failing to inhibit combustion in vertical flame spread tests (VFSTs). All three PAAs have been proven to form strong interactions in water with MMT via their protonated tert‐amine groups. The presence of 12.5 % MMT did not significantly change the thermal and thermo-oxidative stability of PAA coatings, while 2 % MMT add-on affected those of both untreated and PAA-treated cotton fabrics. In HFSTs, substituting 2 % MMT for PAA did not significantly change the flame retardant efficacy of the coatings. In VFSTs, the 2 % MMT/14 % PAA combination inhibited cotton ignition, regardless of the PAA structure, while in the 2 % MMT/11 % PAA combination M-GLU protected cotton from ignition, M-ARG extinguished the flame and M-GLY burned completely. By further reducing the PAA content to 8 %, only M-GLU quenched cotton combustion, leaving an RMF of 82 %. The fact that neither MMT nor PAAs alone induced flame extinguishment at the same add-ons used in the adopted formulations suggests a synergistic behavior of MMT and PAAs.
Introduction
Flame retardants (FRs) for textiles have been studied since the 1950s. Due to the numerous fields of application of textiles, including protec- tive garments, automotive, transportations, they are still of industrial relevance. FRs for cotton have been the most widely investigated [1–7].
Inorganic nanoparticles have emerged as promising flame retardant candidates, especially when applied as coating on textiles [8,9]. One of their advantages, compared to traditional FRs, is the small amount of material required to effectively coat the fabrics and exert their function. In addition, they can be applied using different deposition procedures, including nanoparticle adsorption, in situ synthesis of nanoparticles through sol-gel process [10–15], and Layer-by-Layer (LbL) deposition [16–19]. The mechanism of action of flame retardant nanocomposite coatings, as in bulk nanocomposites, is based on the accumulation, during the combustion process and consequent overheating of textile, of nanoparticles on the coating surface, forming an inorganic barrier that protects the underlying polymer and favors char formation [20–23]. Montmorillonite (MMT), a multilayered aluminosilicate clay bearing sodium or calcium ions in the interlaminar space, is a widely used nanosized inorganic filler present in the flame retardant formulations for polymers. During burning, MMT produces an SiO2 covering, which plays a dual role of insulating and shielding the polymer. This barrier prevents heat, oxygen, and mass transfer, altering the degradation pathways of polymers, and limiting the mobility of polymer chains [24,25]. MMT can help to reduce heat release, smoke release, and the rate of production of toxic gases from composites during burning [26–28]. [Here the full article]